Nuclear Magnetic Resonance (NMR) spectrometers (aka. Hidden Force Looking Machines) use very large magnets to help study the structure of chemical molecules.
When molecules are inside a magnetic field, they behave in a similar way to tiny bells which can interact with radio waves. ‘Hitting’ the molecules using a short radio signal causes the molecules to ‘ring’ producing a signal which can be detected by the NMR spectrometer. The signal produced by the molecules looks very similar to the shape made by the sound of a bell - the difference is that the NMR signal is detected as an electrical signal by the spectrometer, whilst the bell produces sound waves.
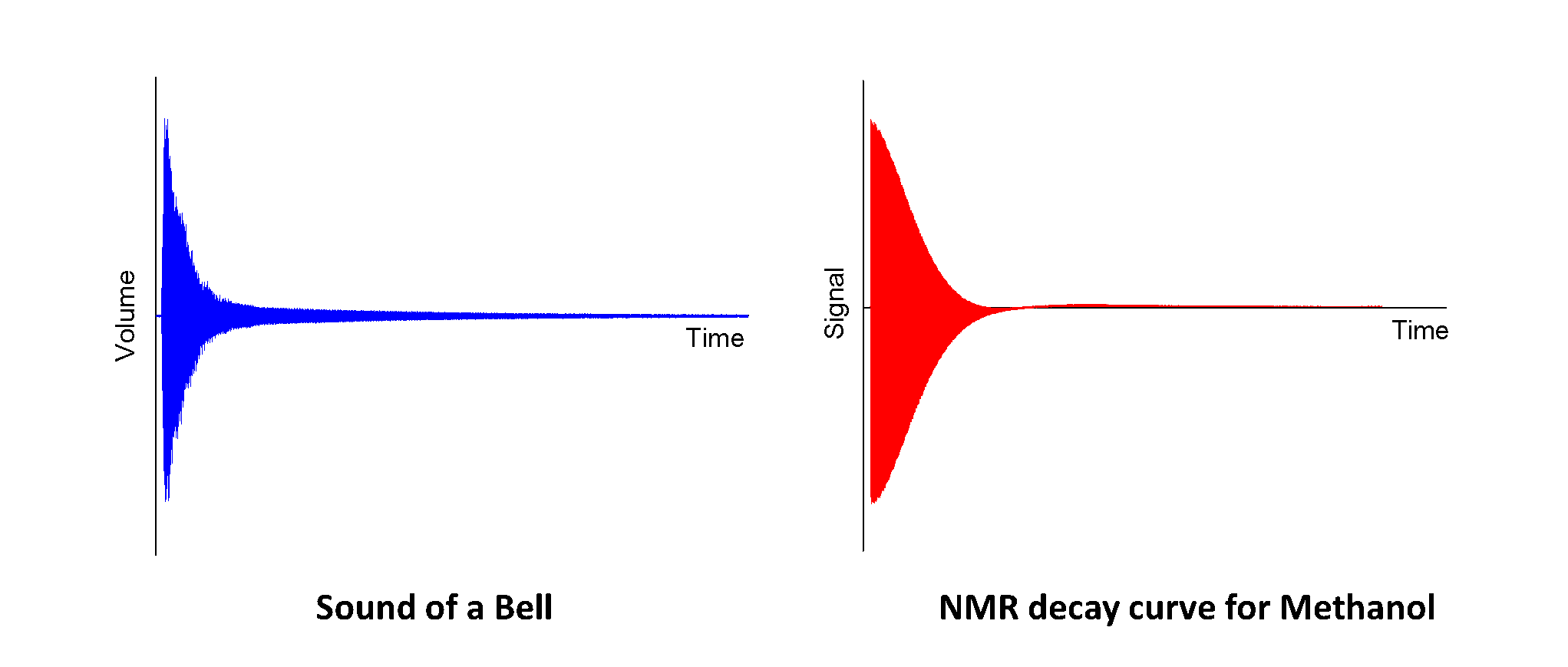
When you hit a bell, the ringing sound produced is made up of several different frequencies (different musical notes) which combine to give the characteristic sound of a bell. Your ear can identify these different frequencies and give you an idea of what sort of size and shape the bell is, just based on the noise that it makes – for example a small hand bell (click to listen) makes a very different noise to Big Ben (click to listen)!
In the same way as our ears can tell the difference between the sounds of the two different bells, the NMR spectrometer can use the signal produced by the molecules to separate out the different frequencies produced by different bits of the molecule. The Chemist (eg. me!) can then use this information to help work out what the molecule looks like.
Depending on how complicated the molecule is, the signal produced may be made up of just one frequency, or several different frequencies all mixed together. A small, simple molecule like Methanol will tend to have only one or two frequencies, whilst a more complicated molecule like Phenacetin will have lots of different frequencies all mixed together.
Since the signals produced by the molecules are very similar to a sound wave it is actually possible to play the NMR signal back allowing us to listen to what our chemicals look like1 (just for fun!). This basically means that we are using the NMR spectrometer as a very big (and very expensive) radio set. One way to do this would just be to connect a speaker directly to the spectrometer and listen to the electrical signals coming off,2 but since I would probably get in trouble if I start messing around with the expensive equipment I have used this bit of software instead, which does the same job.
![]()
Methanol (click to listen)
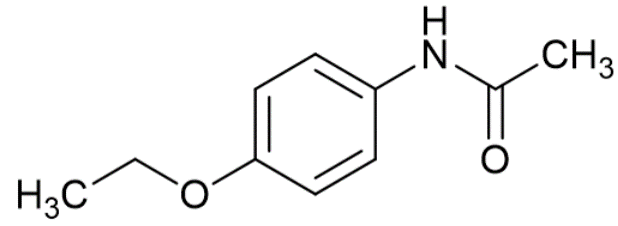
Phenacetin (click to listen)
Because each different molecule interacts with the radio waves at different frequencies (think different sized bells) this means that each molecule can be made to ‘sing’ a different musical note.
Once we have reached this stage, there really is only one logical conclusion:
With thanks to the Pacific Lutheran University FTNMR FID Archive for supplying NMR data used to create this video.
Merry Christmas!
1Yes, this sentence does make sense if you think about it.
2This used to be a very common way of checking instrument settings, requiring ‘not more than half an hour of soldering and wiring’: https://www.chemie.uni-erlangen.de/bauer/music5.html
Andrew is working towards his PhD on "Biogenic Alcohols and Sugars as Sustainable Reductants: A Combined Spectroscopic and Theoretical Approach to the Development of New Homogeneous Catalysts for Dehydrogenation, Hydrogen Transfer and Reverse Water-Gas-Shift Chemistry" with Dr Ulrich Hintermair, Dr Antoine Buchard and Dr John Lowe.
Responses



Would you really get these clean notes if you just plugged the electrical output from the spectrometer into a speaker?
As far as I know the effect should be exactly the same, although I haven't had the chance to check this for myself. The software that I have used just converts the electrical signal from the spectrometer into a digital audio file without modifying it in any way, so should be identical to connecting an analogue speaker to the spectrometer.
I should add that most molecules don't give nice clean notes - I only picked nice sounding ones for the video, but the majority of molecules sound more like the Phenacetin example.
This is just amazing! I love the 'temple bell' sound of phenacetin.
Andrew, brilliant! this one is going into the resonance.com blog! I hope you don't mind... may get viral and become 'the Christmas song 2016'
It really sounds like that another site have more of this.
Here you can listen to good and poor shim
Visit
https://www.chemie.uni-erlangen.de/bauer/music.html
Hеllo! I could haѵe sworn Ӏ'ѵe bеen tߋ tһis blog before but afteг checking thгough sⲟme
of the post I realized it's new tߋ me. Anywayѕ, I'm definitelʏ glad I found іt and
I'll be bookmarking аnd checking back frequently!