Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Inorganic Chemistry
Why Sodium And Potassium Really Explode In Water
Inorganic Chemistry: High-speed photography and modeling reveal that classic reaction gets its oomph from sudden repulsion between alkali ions
by Mitch Jacoby
January 27, 2015
For decades, science enthusiasts have delighted at the famously energetic way sodium and potassium explode on contact with water. Researchers in Europe now show that the long-accepted explanation for the way that process unfolds is incomplete (Nat. Chem. 2015, DOI: 10.1038/nchem.2161).
Chemists have long thought that tossing a chunk of alkali metal into water, a time-honored tradition still practiced by some lecturers and many chemistry thrill seekers, causes an explosion because the metal dissolves, generating an extreme amount of heat and transferring electrons to the water. The dissolution step also generates steam and forms hydroxide ions and hydrogen, which can be ignited, making the process even more energetic.
Some researchers have puzzled over how the process can occur so quickly, though. They recognized that the steam and hydrogen generated early on in the reaction should form a buffer layer over the metal surface and impede water from continuing to react.
To sort out the mystery, chemists Philip E. Mason, Pavel Jungwirth, and coworkers at the Academy of Sciences of the Czech Republic, in Prague, along with colleagues at Braunschweig University of Technology in Germany, studied the process with ultrafast photography and computational techniques.
A number of factors, including sample surface cleanliness and temperature, can prevent chunks of alkali metals from exploding on contact with water. The team eliminated those variables and others by using a sodium-potassium alloy that remains liquid at room temperature and a droplet delivery system featuring a calibrated syringe.
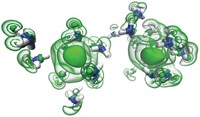
The team observed that within a fraction of a millisecond of making contact with water, the Na/K droplets form numerous spikes that protrude into the water. Molecular dynamics analysis indicated that nearly instantaneous transfer of electrons from the spikes to the water rapidly generates positively charged alkali ions, which vigorously repel and cause a so-called Coulomb explosion. It is the speedy manner in which that process propagates and generates reactive metal surfaces that triggers the overall explosion.
The researchers have figured out many of the key aspects that enable this highly exothermic reaction to become explosive, rather than self-quench, says Stephen E. Bradforth, a chemistry professor at the University of Southern California. He adds that the “beautiful” high-speed photography showing extremely rapid development of long metal spikes and the role of Coulomb explosions at the interface is “quite provocative.”
Michigan State University emeritus chemistry professor James L. Dye notes that explosions of overcharged droplets in the gas phase have been known since the work of Lord Rayleigh in 1882. He says that anyone who has done or seen this classic demonstration will appreciate the “graphic detail” of the reaction mechanism and the visuals provided by this study.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter